I hate to say it, but what I’m about to tell you is nothing new.
The unnecessary suffering I’m about to describe has occurred countless times due to a culture of habit in the intensive care unit, and a lack of support or investment in evidence-based practices.
Regrettably, for many who end up in the ICU, this use of obsolete practices results in a domino effect, where one outmoded practice leads to complications, which are further exacerbated by another outmoded practice, and so on, and so forth.
The story I’m about to tell is one of the worst examples of this domino effect I’ve ever seen, and the unfortunate victim was a man named Jim.
This is his story.
How a Misguided Attempt to Reduce ICU Complications Caused Jim to Suffer Needlessly
Jim is a 70-year-old man with a history of bipolar disorder, generalized anxiety, and previous knee replacement. He has retired from the hockey industry and enjoys being an active part of the lives of his children and grandchildren.
Sadly, he ended up suffering a C6-C7 vertebrae compression fracture and had to undergo cervical decompression surgery. He was discharged from the hospital on Friday, May 15th, 2021.
He returned to the ER on May 16th due to uncontrolled pain. He was given IV morphine, which led to respiratory failure and witnessed cardiac arrest. He was immediately and successfully resuscitated with CPR and then intubated and put on the ventilator.
According to his family, it is unclear if his neurological status was assessed. His MRI did not show evidence of any hypoxic injury during his cardiac arrest. Presumably, Jim was immediately started on continuous sedation solely because he was intubated and on mechanical ventilation.
His family is unaware of any sedation vacations that took place as he remained on mechanical ventilation for another two days. On May 18th, he was extubated and was found to be very combative and physically aggressive with the staff and his family. At this point, Jim was re-intubated and automatically re-sedated.
On May 20th he was extubated again and was again agitated, restrained, confined to the bed, and required a high-flow nasal cannula with a FiO2 of 80%. He was then re-intubated and re-sedated again.
On May 25th (after nine days of mechanical ventilation, sedation, and immobility) he developed ventilator-associated pneumonia. His ventilator settings increased, and his family was called to come to the bedside to prepare for his demise. Fortunately, he responded to antibiotics and survived.
Then, on June 7th, after 22 days of mechanical ventilation, sedation, and immobility, he again achieved ventilator settings of a PEEP 5 and a Fi02 of 40%. He was extubated and remained off the ventilator overnight. He gradually became more agitated, more confused, and developed hypercarbia. At one point, he told his wife, “I can’t breathe. It’s too hard to breathe!” and requested that she push on his diaphragm to help him breathe.
On June 8th he was re-intubated for hypercarbia and respiratory failure (likely due to diaphragm dysfunction), and once again, he was automatically re-sedated.
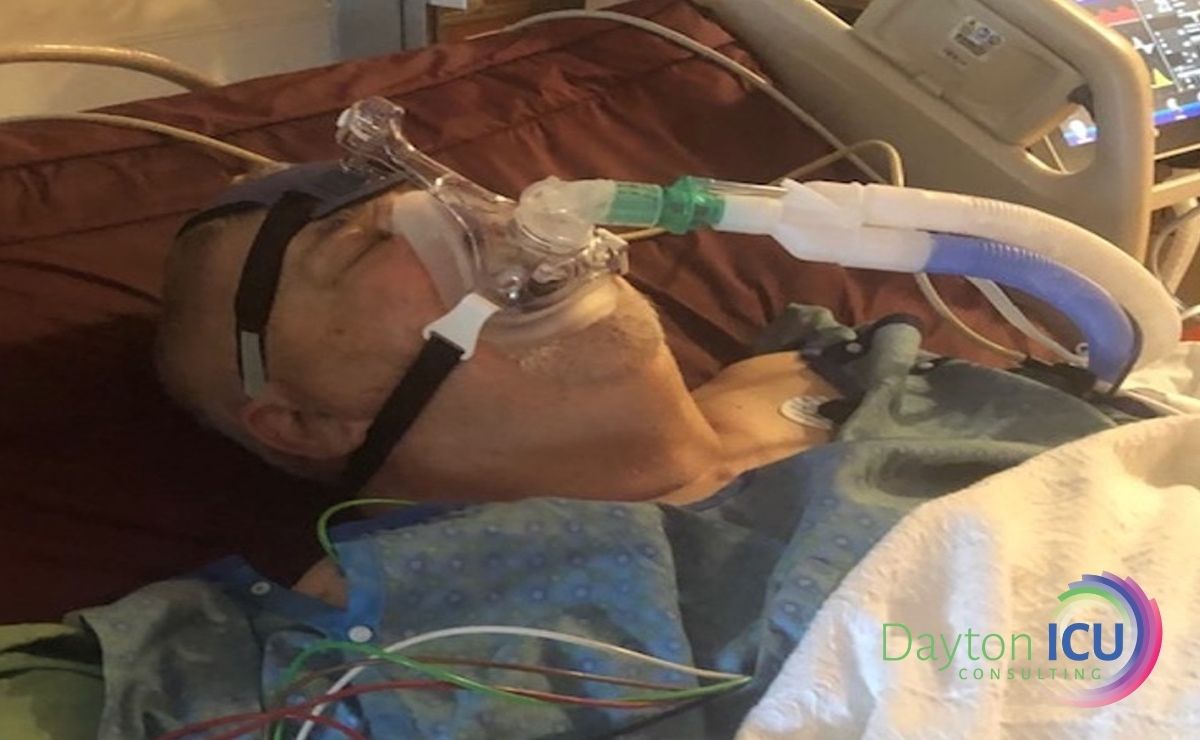
By June 16th he had developed a pulmonary embolism for a lower extremity deep vein thrombosis and required IV anticoagulation. After one month of almost continuous sedation and immobility, he underwent a tracheostomy and PEG tube placement, and sedation was finally discontinued.
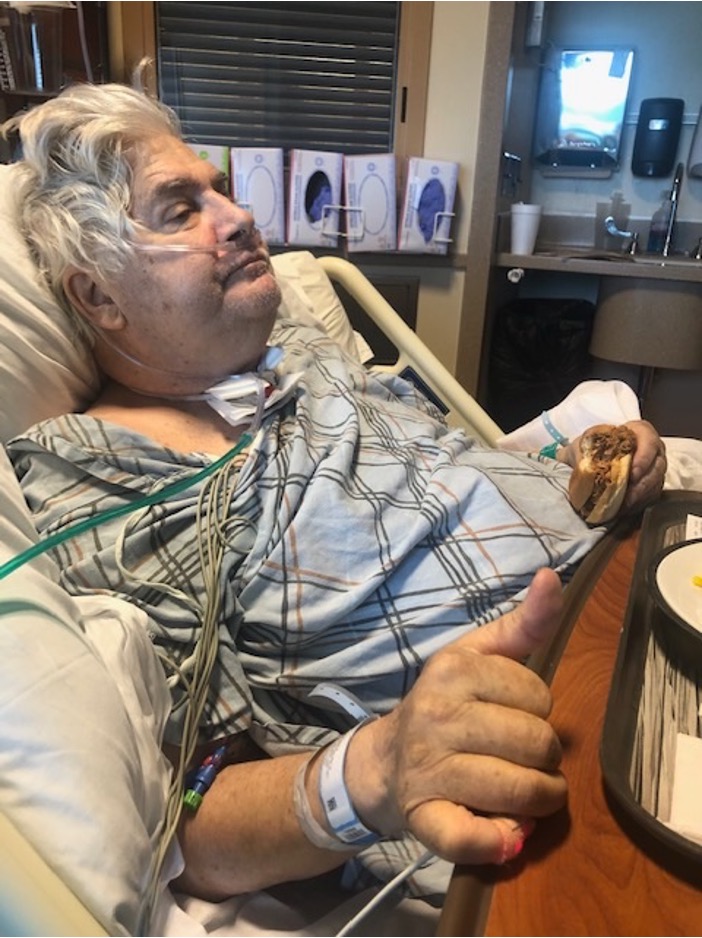
On June 17th he was sent to the step-down unit. Shortly later, he developed an ileus, his PEG tube was dislodged, and he suffered a new infection from his PEG tube complications. He then underwent emergency surgery to replace the PEG tube and was readmitted to the ICU.
Less than 24 hours after being readmitted to the ICU, he was transferred to a long-term acute care hospital (LTACH).
During the following months, he continued to suffer from persistent vomiting from the PEG tube, along with horrific delirium, and struggled to spend more than a few hours off of the ventilator due to his profound weakness and diaphragm dysfunction.
He developed MRSA as a second ventilator-associated pneumonia. After months of aggressive rehabilitation, he was eventually able to be weaned off of the ventilator and have his tracheostomy removed.
He was discharged home on August 11th and was cared for by his wife and home health caregivers.

He continues to suffer cognitive impairments and unspeakable PTSD, and is unable to participate in the puzzles and activities he used to enjoy.
He refuses to talk about the terrors he experienced under sedation.
Fortunately, as of January 2022, his bipolar disorder is once again under control. He has had two more hospital readmissions, but his family reports that he is finally stabilizing and progressing at home.
The photo below shows Jim’s first time back on his lawn mower, about six months after his admission to the ICU, as he’s finally starting to resume his life again.

Jim’s daughter, Leah, provided a perfectly unfortunate demonstration of how automatically starting sedation after intubation hits the first domino of this entire chain of consequences. She shares her father’s journey in Episode 81 of my Walking Home From The ICU podcast.
What Went Wrong: How Things Could Have Been Different for Jim and the ICU Team Members Who Treated Him
Jim’s journey clearly demonstrates the impact of the decision to start continuous sedation after every intubation.
If Jim had been allowed to wake up immediately after his cardiac arrest, it is likely that he may have been able to be extubated and discharged home from the hospital shortly thereafter.
But as a result of automatically starting sedation without a clear indicator, Jim was forced to stay on the ventilator when it may not have been necessary.
He was subjected to terrible delirium, as manifested by his agitation and combativeness when sedation was discontinued after two days. But instead of recognizing and treating his delirium, he was automatically re-intubated for his delirium, and the very sedation that caused the delirium was resumed.
By being denied the opportunity to stay physically strong and functional on the ventilator, he likely developed diaphragm dysfunction, as well, in addition to his delirium. This probably contributed to his need for a high-flow nasal cannula after his second extubation.
This diaphragm dysfunction became even more evident after his third extubation following 22 days of immobility and sedation. He reported he was unable to breathe and required a third intubation ventilator support again, despite minimal ventilator settings.
This is a prime example of how sedation and immobility cause patients to be on the ventilator for days or months longer than if they had been awake and mobile while ventilated.
The sedation and immobility also led him to suffer from three hospital-acquired infections, including two ventilator-associated bouts of pneumonia, and a PEG tube infection.
Oftentimes, this is not even taken into consideration, but the fact of the matter is the more patients are intubated, reclined, immobile, and unable to cough, the more at risk they are of developing ventilator-associated pneumonia.
Had Jim been allowed to wake up after intubation and promptly extubated, he would not have needed the ventilator or the PEG tube in the first place. These infections led to him being readmitted to the ICU, spending more time on the ventilator, more time in the hospital, and ultimately, enduring more suffering.
Jim was discharged on anticoagulation for the pulmonary embolism he developed, which was likely caused by his month of immobility. He was later readmitted to the hospital for bleeding complications due to his long-term anticoagulation.
On the tenth day of his ICU stay, Jim’s family started to advocate for sedation cessation and early mobility. Unsurprisingly, they were constantly told that the ICU team “had neither the time nor resources” for those interventions.
The painful irony is that their failure to invest time and resources into evidence-based practices for patients like Jim ended up costing the ICU team a month’s worth of time, money, staff, and resources providing care that was probably unnecessary.
This should provide a poignant reminder to all ICU teams that poor patient care is costly, both for them and their patients.
To summarize, Jim came to the ER for postoperative back pain, and he became too sedated following IV morphine, which led to his cardiac arrest and prompt resuscitation.
Sadly, a simple neurological exam and evaluation of the need for mechanical ventilation soon after intubation and throughout his time in the ICU could have drastically changed the trajectory of his life, which would have benefited everyone, including him, his family and the ICU team members who treated him.
Nothing But The Facts: How Evidence-Based Practices Can Reduce ICU Complications, and What Can Happen When They’re Not Applied
The research is clear.
Sedation increases the risk of:
- Dying in the ICU or after the ICU [1, 2]
- Infection [3, 4]
- Pressure sores [5]
- Blood clots [6]
- Delirium [7, 8, 9]
- ICU-acquired weakness [10]
- More time on the ventilator [11]
- More time in the hospital [12]
- Tracheostomy [13]
- Discharge from hospital to a rehab center or nursing home [14]
- Post-ICU PTSD [15, 16]
- Post-ICU dementia (cognitive dysfunction) [17]
- Depression [18]
- Being readmitted to the hospital and ICU [19]
- Post-intensive care syndrome [20]
Sedation decreases the chances of:
- Discharging home from the hospital [14]
- Being able to walk upon transfer from the ICU [21]
- Returning to work [22]
- Having an optimal quality of life [23]
Avoiding sedation and ensuring early mobility, as recommended in the ABCDEF Bundle, decreases the risk of:
- Death [24]
- Ventilator and hospital-associated pneumonia [25]
- Central line and catheter infections [26]
- Pressure injuries [27]
- Falls [28, 29]
- Delirium [24]
- Aspiration pneumonia [30]
- Constipation/ileus [31]
- Intubation [14]
- Re-intubation [14]
- Tracheostomy and PEG tube placements [32]
- Discharge to care facilities [24]
- Hospital and ICU readmissions [24]
- Diaphragm dysfunction [33]
- ICU-acquired weakness [34]
These practices can also improve the chances of:
- Successful extubation [24]
- Discharges from ICU [24]
- Discharges home [24, 35]
- Survival [24]
- Functional independence after discharge [36]
- Having optimal quality of life [37]
- Proper lung aeration [38]
- Secretion clearance [39]
The choice to automatically sedate Jim after intubation and the failure to apply evidence-based practices in his care, such as the ABCDEF bundle, ended up resulting in:
- Weeks and/or months of ICU delirium
- Diaphragm dysfunction
- ICU-acquired weakness
- Deep vein thrombosis (blood clots)/pulmonary embolism
- Three failed extubations
- Three intubations
- A tracheostomy
- Two cases of ventilator-associated pneumonia
- A total of three hospital-acquired infections
- Readmission to the ICU
- A preventable one-month ICU stay
- Danger and burden to ICU staff
- Months of painful and difficult rehabilitation in a LTACH
- Post-ICU PTSD
- Post-ICU dementia
- Bleeding complications from long-term anticoagulation
- Trauma and burden to family caregivers
- Decreased quality of life
- Two hospital readmissions during the first five months following discharge home
Even while he was in excruciating pain following back surgery, Jim walked into the hospital, and he should have received the care to be able to walk himself out.
Unfortunately, the failure of so many ICUs to implement these evidence-based practices creates an unimaginable amount of unnecessary suffering, and burdens patients like Jim, their families, and the ICU teams in the hospitals where they’re treated.
But things do not have to be this way. With the implementation of evidence-based practices, such as the ABCDEF bundle, ICUs around the world can move beyond the archaic care they’re currently providing and do what’s best for themselves and their patients.
Are you looking to learn more about how evidence-based practices can reduce ICU complications, and how you can work to implement them in your ICU? I can walk you through the entire process, so please don’t hesitate to contact me.
References:
- Shehabi, et al. (2012). Early intensive care sedation predicts long-term mortality in ventilated critically ill patients. American Journal of Respiratory and Critical Care Medicine, 186(8), 724-731.
- Tanaka, et al. (2014). Early sedation and clinical outcomes of mechanically ventilated patients: a prospective multicenter cohort study. Critical Care (London, England), 18(4), R156.
- Lin, et al. (2008). Risk factors for the development of early-onset delirium and the subsequent clinical outcome in mechanically ventilated patients. Journal of Critical Care, 23(3), 372-379.
- Rello, J., Diaz, E., Roque, M., & Vallés, J. (1999). Risk factors for developing pneumonia within 48 hours of intubation. American Journal of Respiratory and Critical Care Medicine, 159(6), 1742-1746.
- Cox, J., Roche, S., & Murphy, V. (2018). Pressure Injury Risk Factors in Critical Care Patients: A Descriptive Analysis. Advances in Skin & Wound Care, 31(7), 328-334.
- Minet, C., Potton, L., Bonadona, A., Hamidfar-Roy, R., Somohano, C. A., Lugosi, M., Cartier, J. C., Ferretti, G., Schwebel, C., & Timsit, J. F. (2015). Venous thromboembolism in the ICU: main characteristics, diagnosis and thromboprophylaxis. Critical Care (London, England), 19(1), 287.
- Pandharipandh, P., Shintani, A., Peterson, J., Truman, B., Wilkinson, G., Dittus, R., Bernard, G., Ely, W. (2006). Lorazepam is an independent risk factor for transitioning to delirium in intensive care unit patients. Anesthesiology, 104. https://pubs.asahq.org/anesthesiology/article/104/1/21/7483/Lorazepam-Is-an-Independent-Risk-Factor-for
- Yang, J., Zhou, Y., Kang, Y., Xu, B., Wang, P., Lv, Y., & Wang, Z. (2017). Risk Factors of Delirium in Sequential Sedation Patients in Intensive Care Units. BioMed Research International, 2017, 3539872.
- Pereira, J. V., Sanjanwala, R. M., Mohammed, M. K., Le, M. L., & Arora, R. C. (2020). Dexmedetomidine versus propofol sedation in reducing delirium among older adults in the ICU: A systematic review and meta-analysis. European Journal of Anaesthesiology, 37(2), 121-131.
- Vanhorebeek, I., Latronico, N., & Van den Berghe, G. (2020). ICU-acquired weakness. Intensive Care Medicine, 46(4), 637-653.
- Shehabi, et al. (2012). Early intensive care sedation predicts long-term mortality in ventilated critically ill patients. American Journal of Respiratory and Critical Care Medicine, 186(8), 724-731.
- Strøm, T., Martinussen, T., & Toft, P. (2010). A protocol of no sedation for critically ill patients receiving mechanical ventilation: a randomised trial. Lancet (London, England), 375(9713), 475-480.
- Brook, A. D., Ahrens, T. S., Schaiff, R., Prentice, D., Sherman, G., Shannon, W., & Kollef, M. H. (1999). Effect of a nursing-implemented sedation protocol on the duration of mechanical ventilation. Critical Care Medicine, 27(12), 2609-2615.
- Pun, et al. (2019). Caring for Critically Ill Patients with the ABCDEF Bundle: Results of the ICU Liberation Collaborative in Over 15,000 Adults. Critical Care Medicine, 47(1), 3-14.
- Davydow, D. S., Gifford, J. M., Desai, S. V., Needham, D. M., & Bienvenu, O. J. (2008). Posttraumatic stress disorder in general intensive care unit survivors: a systematic review. General Hospital Psychiatry, 30(5), 421-434.
- Nelson, B. J., Weinert, C. R., Bury, C. L., Marinelli, W. A., & Gross, C. R. (2000). Intensive care unit drug use and subsequent quality of life in acute lung injury patients. Critical Care Medicine, 28(11), 3626-3630.
- Wilcox, M. E., Brummel, N. E., Archer, K., Ely, E. W., Jackson, J. C., & Hopkins, R. O. (2013). Cognitive dysfunction in ICU patients: risk factors, predictors, and rehabilitation interventions. Critical Care Medicine, 41(9 Suppl 1), S81-S98.
- Desai, S. V., Law, T. J., & Needham, D. M. (2011). Long-term complications of critical care. Critical Care Medicine, 39(2), 371-9.
- Tanaka, et al. (2014). Early sedation and clinical outcomes of mechanically ventilated patients: a prospective multicenter cohort study. Critical Care (London, England), 18(4), R156.
- Rawal, G., Yadav, S., & Kumar, R. (2017). Post-intensive care syndrome: An overview. Journal of Translational Internal Medicine, 5(2), 90-92.
- Vanhorebeek, I., Latronico, N., & Van den Berghe, G. (2020). ICU-acquired weakness. Intensive Care Medicine, 46(4), 637-653.
- Cox, J., Roche, S., & Murphy, V. (2018). Pressure Injury Risk Factors in Critical Care Patients: A Descriptive Analysis. Advances in Skin & Wound Care, 31(7), 328-334.
- Nelson, B. J., Weinert, C. R., Bury, C. L., Marinelli, W. A., & Gross, C. R. (2000). Intensive care unit drug use and subsequent quality of life in acute lung injury patients. Critical Care Medicine, 28(11), 3626-3630.
- Mart, M., Brummel, N., & Ely, W. (2019). The ABCDEF Bundle for the Respiratory Therapist. Respiratory Care, 64(12).
- Clark, et al. (2013). Effectiveness of an early mobilization protocol in a trauma and burns intensive care unit: a retrospective cohort study. Physical Therapy, 92(2).
- Hunter, A., Johnson, L., & Coustasse, A. (2014). Reduction of intensive care unit length of stay: the case of early mobilization. Health Care Management, 33(2).
- Azuh, et al. (2016). Benefits of early active mobility in the medical intensive care unit: a pilot study. American Journal of Medicine, 129(8).
- Hshieh, et al. (2015). Effectiveness of multicomponent nonpharmacological delirium interventions: a meta-analysis. JAMA Internal Medicine, 175(4).
- Centers for Medicare & Medicaid Services. (2017). Mobility Action Group: Change package and toolkit [White paper]. U.S. Department of Health & Human Services.
- d’Escrivan, & Guery, B. (2005). Prevention and treatment of aspiration pneumonia in intensive care units. Treatments in Respiratory Medicine, 4.
- Sutton, et al. (2021). Impact of the 2018 society of critical care medicine pain, agitation/sedation, delirium, immobility, and sleep guidelines on nonopioid analgesic use and related outcomes in critically ill adults after major surgery. Critical Care Explorations, 3(10).
- Brook, et al. (1999). Effect of a nursing-implemented sedation protocol on the duration of mechanical ventilation. Critical Care Medicine, 27(12).
- Dong, et al. (2021). Early rehabilitation relieves diaphragm dysfunction induced by prolonged mechanical ventilation: a randomized control study. BMC Pulmonary Medicine, 21(106).
- Lipshutz, A., & Gropper, M. (2013). Acquired neuromuscular weakness and early mobilization in the intensive care unit. Anesthesiology, 118(1).
- Ota, et al. (2015). Effect of early mobilization on discharge disposition of mechanically ventilated patients. Journal of Physical Therapy Sciences, 27(3).
- Schujmann, et al. (2019). Impact of a progressive mobility program on the functional status, respiratory and muscular systems of ICU patients: A randomized and controlled trial. Critical Care Medicine 48(4).
- Ely, W. (2017). The ABCDEF Bundle: Science and philosophy of how ICU liberation serves patients and families. Critical Care Medicine, 45(2).
- Elmer, et al. (2021). The effect of physical therapy on regional lung function in critically ill patients. Frontiers in Physiology, 12.
- Strickland, et al. (2013). AARC clinical practice guideline effectiveness of nonpharmacologic airway clearance therapies in hospitalized patients. Respiratory Care, 58(12).


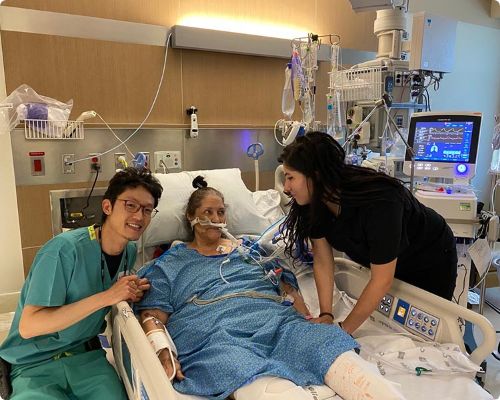 I stumbled upon Kali’s podcast midway through my anesthesia critical care fellowship in February 2021. At our institution, I got the impression that patients in the ICU either got better on their own or had a prolonged and complicated course to LTAC or death. In her podcast, Kali explained that LTAC was rarely the outcome for patients in the Awake and Walking ICU in Salt Lake City.
I stumbled upon Kali’s podcast midway through my anesthesia critical care fellowship in February 2021. At our institution, I got the impression that patients in the ICU either got better on their own or had a prolonged and complicated course to LTAC or death. In her podcast, Kali explained that LTAC was rarely the outcome for patients in the Awake and Walking ICU in Salt Lake City.
