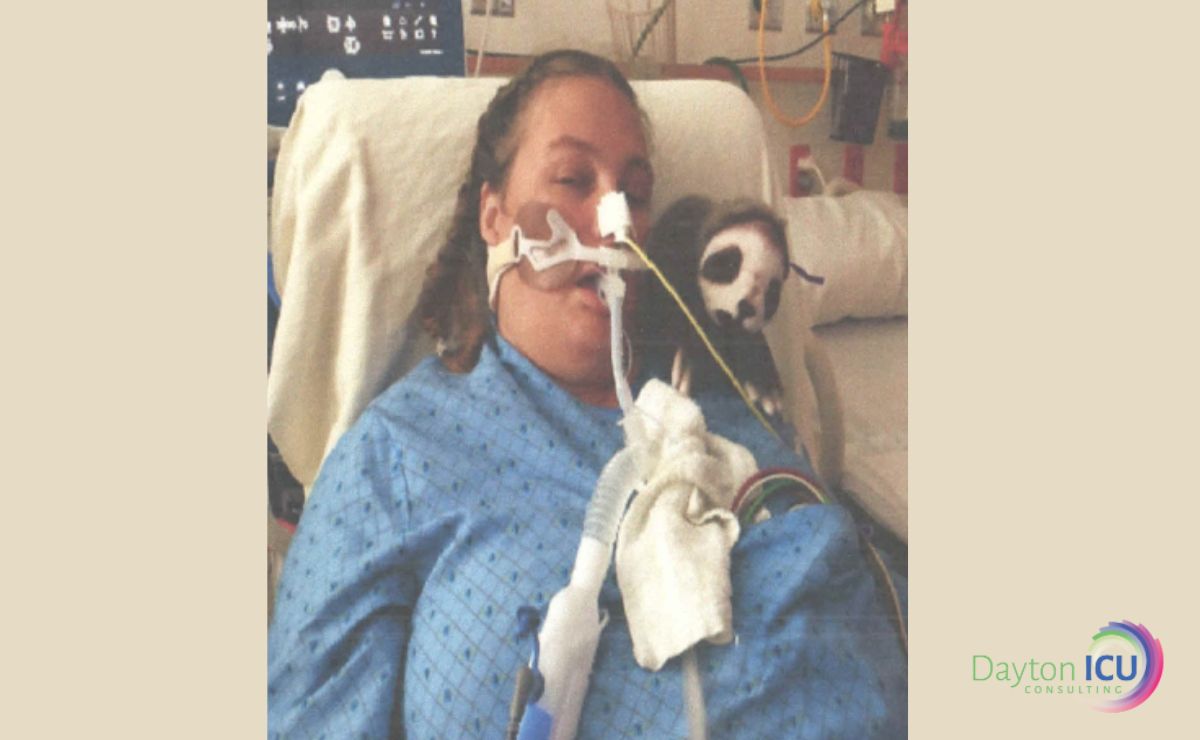
“See? From this we see that mobility does nothing!”
This is the common rebuttal that I have received from at least a few physicians in every single ICU I have trained since the TEAM Study was published in The New England Journal of Medicine in 2022.
There is often a sparkle of mischief in their eye, as if to say, “Gotcha!” like it was some sort of a competition to be won.
They expect my entire mission to be derailed and that they are justified in continuing sedation and immobility as usual.
As far as they’re concerned, they have “proven” that early mobility has zero benefit to outcomes.
When the study was published, one of its authors tweeted, “Rest is best” in reference to its findings.
Is this really the conclusion of the TEAM Study?
The TEAM Study, which is short for Treatment of Mechanically Ventilated Adults with Early Activity and Mobilization, was developed to explore the impact of an increased dose of mobility with the understanding that mobility is feasible and beneficial in the ICU.
The outcomes measured were the number of days that the patients were alive and out of the hospital at 180 days after randomization.
A superficial skim through the conclusion on the abstract does state that “an increase in early active mobilization did not result in a significantly greater number of days that patients were alive and out of the hospital than did the usual level of mobilization in the ICU.”
And a recent financial analysis, written on behalf of the TEAM Study Investigators, concluded that there were no cost-savings associated with higher-dose early active mobilization.
Truth be told, the TEAM Study is full of lessons and take-aways, but if you look at it with a discerning eye, you’ll realize that “mobility does nothing” is not one of them.
In any case, I hosted a group discussion with the author, Carol Hodgson, DPT, as well as Dr. Wes Ely and Heidi Engel, DPT on Episode 117 of my Walking Home From The ICU podcast, where we explored the methodology and what the study ultimately can teach us.
Sedation
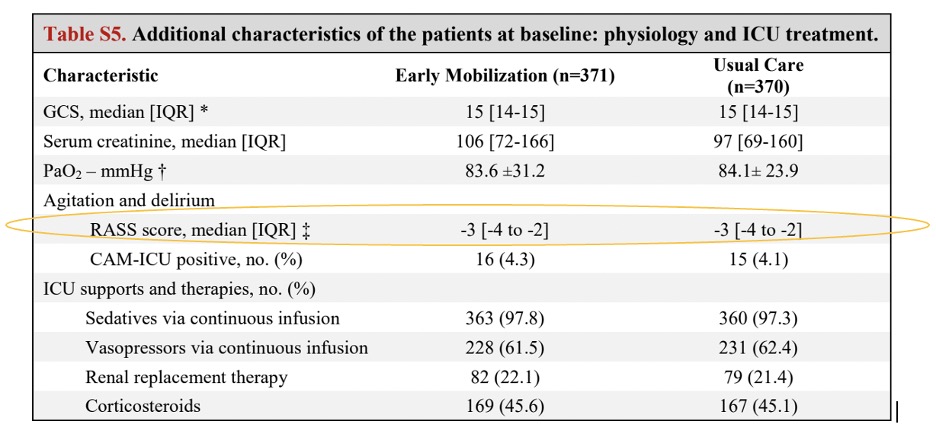
As you can see from the data below, participants in the TEAM Study were all automatically sedated and spent the next six days at a median RASS of -3.
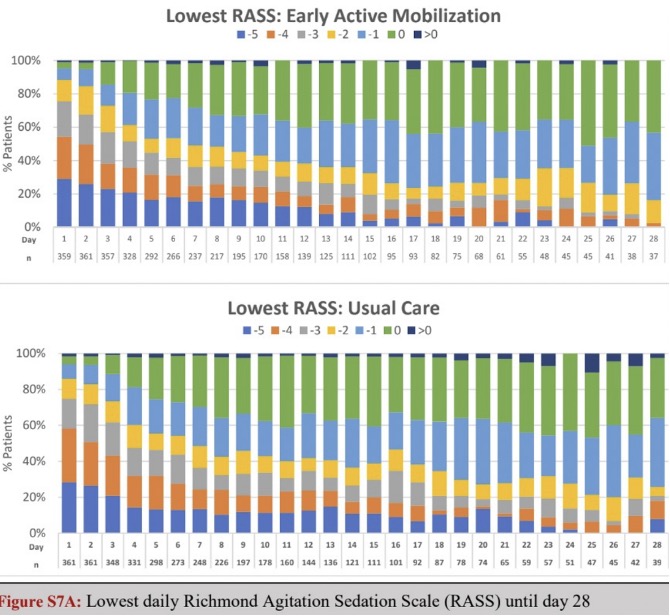
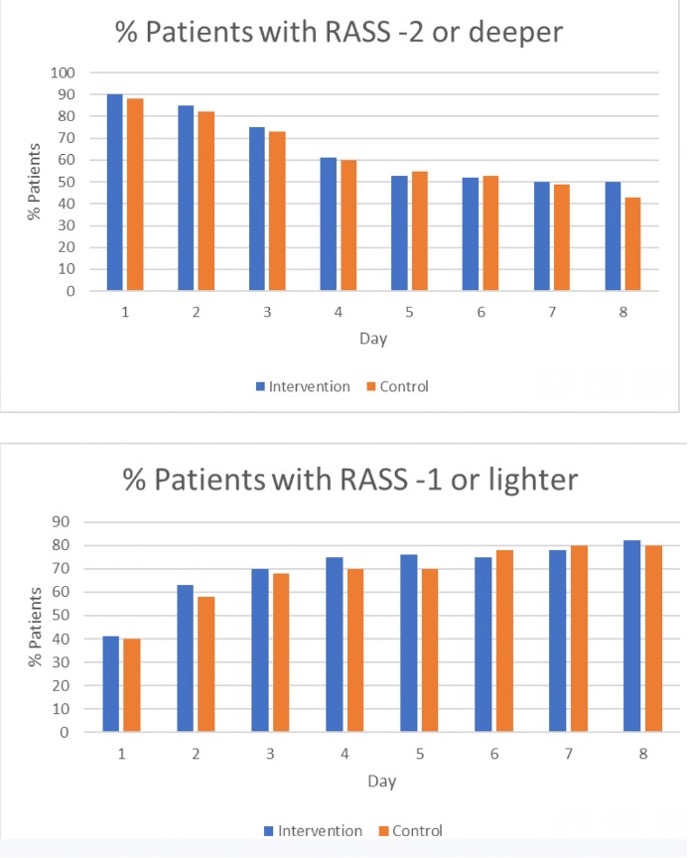 We know that patients lose 2% of muscle mass per day in the ICU and more than 15% per week.
We know that patients lose 2% of muscle mass per day in the ICU and more than 15% per week.
It’s also important to note that muscle function is lost more quickly than muscle mass, especially in the setting of Propofol, which disrupts sodium channels of the muscles and decreases muscle excitability, as well as leading to toxicity and damage to the mitochondria.
Six days of moderate sedation and absolute immobility during critical illness can cause significant muscular damage and loss that will leave ICU clinicians and survivors with the challenge of recovery. Additionally, sedation increases the risk of delirium by 226%.
However, mechanical ventilation is not an indication for sedation.
Continuous sedation (especially to achieve a RASS score of -3) is indicated for certain exceptions, including things like intracranial hypertension, status epilepticus, and the inability to tolerate oxygen consumption, which are all conditions that would disqualify patients from being included in the TEAM Study.
It is therefore concerning that the 750 adult patients in the control and intervention groups would be moderately sedated for six days before starting “sedation minimization.”
This study accurately captures the sedation and immobility conveyor belt that most ICU teams have inherited throughout the years.
This leads to high rates of delirium and ICU-acquired weakness that then become incredible challenges to rehabilitate later.
Timing
My mentor, Polly Bailey, APRN, always said, “What we do on the front end of critical illness determines the back end.”
In other words, how we manage sedation and delirium during the early phases of critical illness will greatly impact what happens days and weeks later.
In an Awake and Walking ICU, mobility is provided promptly as soon as there is no longer an indication for sedation.
My home ICU had the goal of mobilizing patients within 12 hours after admission/intubation.
We see in the research a spectrum of timing for mobility ranging between 1-8 days after ICU admission, yet the studies demonstrating the most benefit mobilized patients early on.
Mobilizing patients within two days after intubation has been proven to improve cognitive function by 20% one year after discharge.
And mobilizing patients within 72 hours after intubation decreased delirium and time on the ventilator by two days and increased return to baseline function by 24% by discharge.
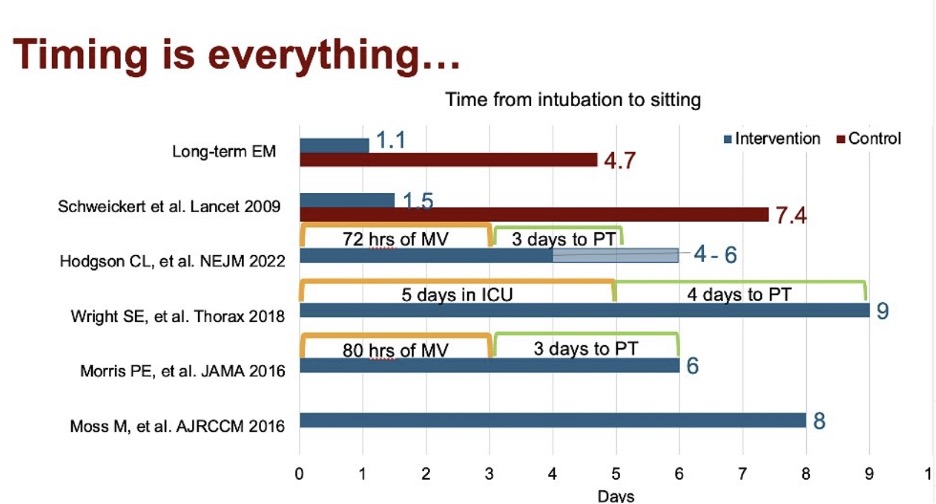
At any rate, if you want to preserve rapidly declining muscle mass and cognitive function in the ICU, early mobility is key. And mobility is most effective when it’s used as a prehabilitation intervention to protect and proactively preserve function.
The TEAM Study also demonstrates the definition of “early” that is utilized in many ICUs.
Typically, ICU clinicians would say something like, “Once ventilator settings are minimal and we’re trying to extubate then we can start to turn down sedation and try to move patients.”
Patients were randomized into the study 2.5 days after admission and mobility was not initiated until three days after that, on average.
Ultimately, this means the rates and severity of delirium and ICU-acquired weakness are increased and mobility is not initiated until rehabilitation is now required.
But if you want it to have any impact on preventing post-ICU syndrome, mobility must be done within 72 hours after ICU admission.
Patients in the ICU are already at high risk of delirium and ICU-acquired weakness, and yet, sedation and immobility are independent risk factors for these very conditions.
We should be striving to prevent ICU delirium and ICU-acquired weakness rather than only addressing these complications after they’ve already happened.
Barriers
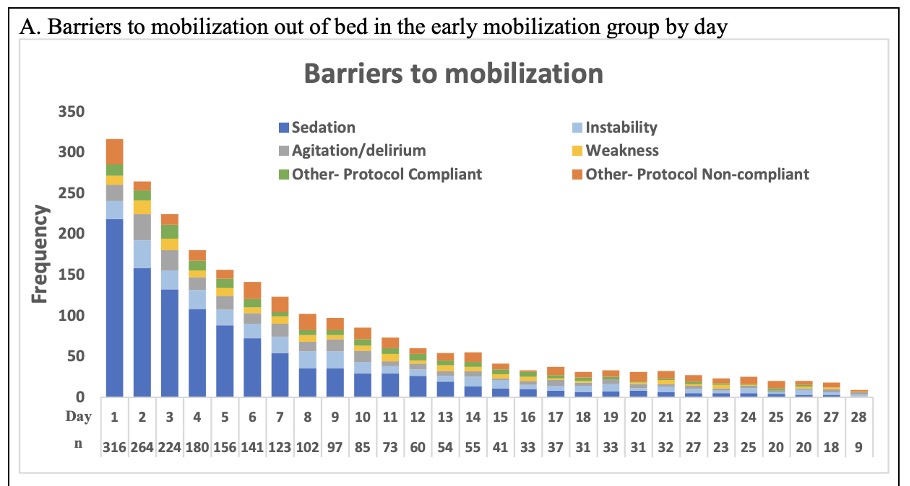
The TEAM Study revealed that the main barriers to mobility were sedation and agitation.
This seems to capture the phenomenon that happens when we automatically run continuous sedation. And when we later try to approach mobility, we often face the following scenarios:
- Challenges and concerns occur when it comes to the timing or convincing of nurses to turn sedation off, such as patients being too sedated to mobilize.
- Sedation is turned off and then patients are still under the effects of sedation for hours or days and cannot engage with mobility.
- When sedation is turned off, patients emerge with agitation from delirium, pain, panic, inability to communicate, etc.
The common response to the third scenario is to resume sedation, which further prevents patients from being able to engage and mobilize.
Considering the high risks of delirium in critically ill patients who have been sedated to a RASS of -3 for six days in this study, we can suspect that delirium may have been a common root of the agitation that prevented mobility for so many patients.
This study also perfectly demonstrates the lack of practicality and feasibility in this approach to sedation and mobility.
And this approach will inevitably lead patients into a vicious cycle, where sedation causes delirium, delirium causes agitation, and then sedation is once again resumed.

Level of Mobility
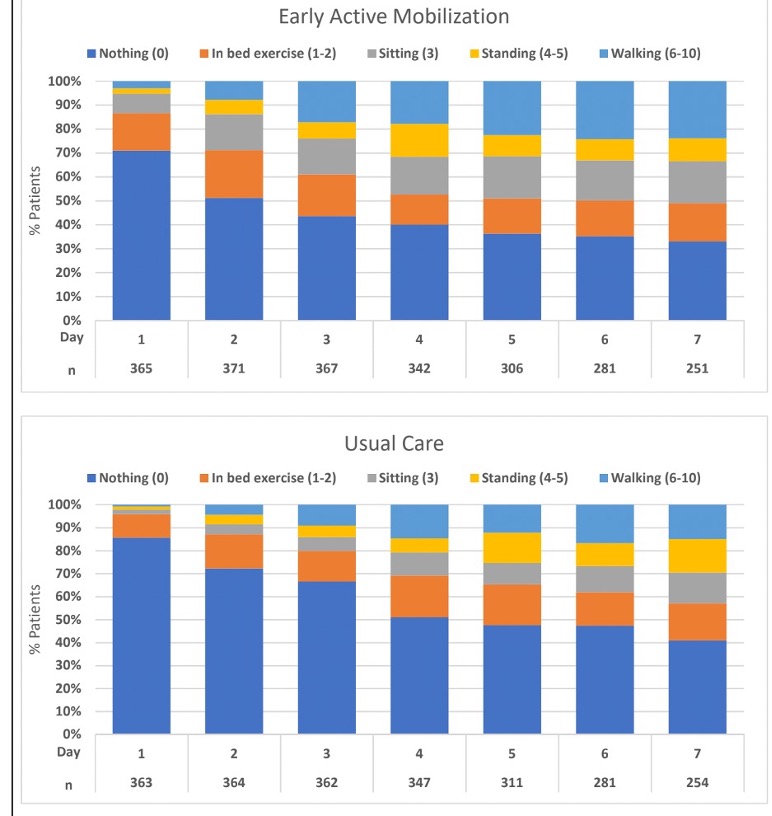
The level of mobility used in early mobility research has been all over the place, including everything from passive range of motion to ambulation and everything in between.
To be fair, the TEAM Study did try to focus on definitive active mobility, although a tilt table still qualified as “standing” in the data collection.
However, less than half of participants in both groups were able to achieve walking, but this didn’t occur until days 4-8 of the study, which would have been days 7-11 after admission.
And about 40 patients in both groups never even achieved sitting at the edge of the bed.
The low level of mobility used in this study may be capturing the loss of mobility that happens during those days of sedation and immobility.
It may also be a result of the challenges of taking a sedation “vacation” briefly for mobility, during which patients are still delirious and/or sluggish from the sedation and are physically and cognitively unable to participate in higher levels of mobility.
Frequency/Dose of Mobility
The limited research that’s been done on dose and frequency of mobility is likely what inspired the development and execution of the TEAM Study.
If we’re going to “treat mobility like an antibiotic” then we should be trying to find an optimal dose, right?
The control group provided eight minutes of active mobility while the intervention group provided 22 minutes.
We do know that there is a substantially positive impact on patient functional outcomes when over 40 minutes of active mobility is provided each day.
More recent studies have shown a dose-dependent relationship.
For instance, in one study, every 10 additional minutes of mobility in the ICU resulted in a 1.2 day decrease in hospital length of stay.
Another study, which I discussed on Episode 173 of Walking Home From The ICU, found that each unit of out of bed mobility decreased time on the ventilator by 10%.
So, the question must be asked: After six days of sedation and immobility, is 14 additional minutes of mobility enough to provide sufficient rehabilitation and make a significant impact on time in the hospital and mortality?
Lack of Contrast Between Control and Intervention Groups
One of the biggest challenges in drawing scientific conclusions from this study is the lack of contrast between the control and intervention groups.
Sedation: Median RASS of -3 for six days in both groups
Timing: Mobility occurred on day six (median) for both groups
Dose: EM 20.8±14.6 vs. UC 8.8±9.0 minutes
Level: When comparing both the control and intervention groups, there was a:
- 0% difference between the number of patients that achieved sitting at EOB
- 1% difference between the number of patients that achieved assisted standing
- 9% difference between the number of patients that achieved assisted walking
Ultimately, we need a second version of this study in which the intervention group of the TEAM Study is used as the control group, and compared to full compliance with the ABCDEF Bundle in an Awake and Walking ICU where sedation is turned off after intubation and patients are promptly performing their highest level of mobility, using a rehabilitative approach for all patients possible.
We will then have a significant enough contrast in timing, dose, and level of mobility to be able to learn from.
Isolating Mobility
The TEAM Study portrays the classic pitfalls of early mobility programs that are attempted in isolation from other elements of the ABCDEF Bundle.
This study only tracked RASS levels, but did not include type of sedation, dose, duration, etc., which is very challenging, as all of the elements of the Bundle interact with and depend on each other.
In any case, I have to question the logic of administering deliriogenic and myotoxic medications for six days and then taking breaks for mobility to likely resume and leave patients immobilized the other 23 or so hours of the day.
We must see mobility as part of a bigger process of care that should be initiated upon admission to the ICU, instead of being an isolated intervention that consists of short daily visits toward the end of critical illness.
Adverse Events
There have also been concerns regarding the adverse events found during the TEAM Study.
As the abstract states, “Adverse events…were reported in 34 of 371 patients (9.2%) in the early-mobilization group and in 15 of 370 patients (4.1%) in the usual-care group (P = 0.005).”
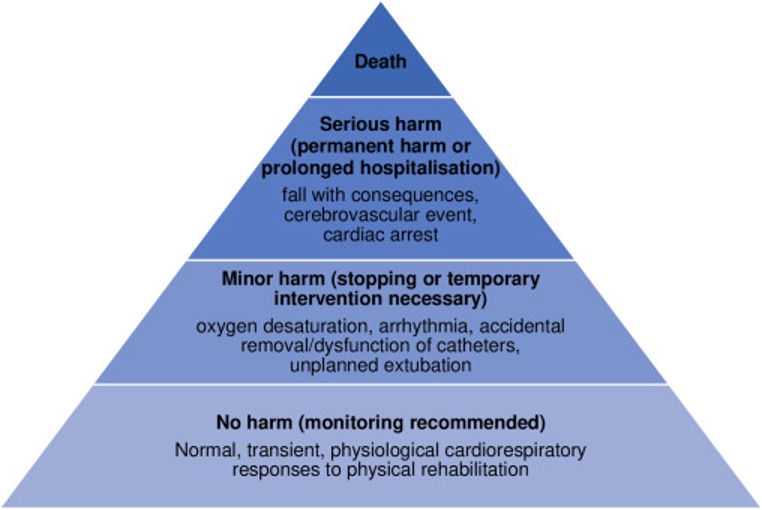
A wonderful rebuttal to this can be found in a must-read eClinicalMedicine article, in which Sabrina Eggmann and company discuss the need to clearly categorize “adverse events” in mobility research.
It is imperative to use proper screening and assessment prior, during, and after mobility to determine if patients are appropriate for starting and continuing mobility.
Nonetheless, Eggmann makes the point that there are normal physiological responses to mobility that are anticipated to occur, including elevated heart and respiratory rate to events causing “minor harm” such as a brief decrease in blood pressure, arrhythmia, intermittent hypoxia, etc.
These events need to be distinguished from truly adverse events and assessed, as “delineation in reporting may help to ensure the balance of risk and benefit is appraised fairly when amalgamating evidence.”
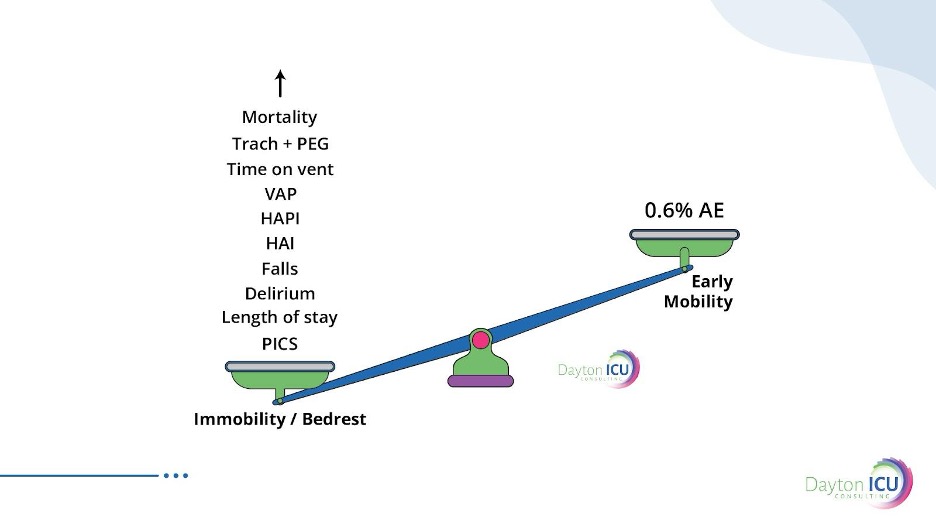
A large meta-analysis with over 14,000 mobility sessions found a 0.6% adverse event rate.
And that analysis included a study from an Awake and Walking ICU that had patients ambulating with a median P/F ratio of 89 and an adverse event rate of 0.6%.
Costs
I have previously shared numerous studies regarding the financial benefits of the ABCDEF Bundle.
These benefits have been measured by looking at things like time on the ventilator, time in the ICU and hospital, readmissions, etc.
At any rate, understanding the lack of contrast between the control and intervention groups in the TEAM Study helps us understand the lack of contrast in outcomes, as well as financial benefits, as shared in a recent Critical Care Medicine article.
I suspect that had the intervention group been compared to care in ICUs that do not mobilize intubated patients, the contrast would have been much more significant.
And if we could compare the intervention group to an Awake and Walking ICU, we would likely see an impressive difference in health-care costs.
Nonetheless, without a true contrast in care, you won’t see a contrast in outcomes or costs.
Case Study
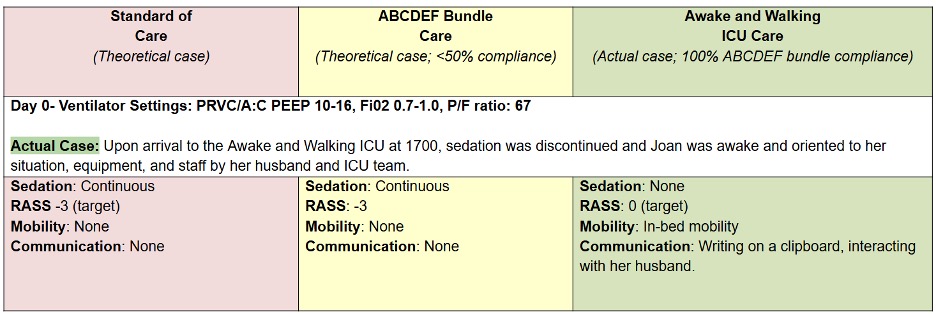
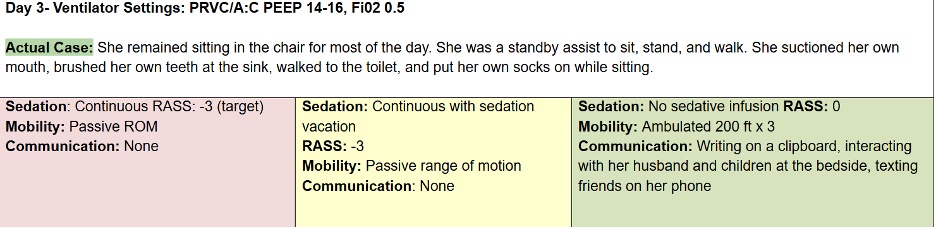
Let’s compare the process of care in the TEAM Study to an Awake and Walking ICU approach, using a real case study, as shared in the following data tables.
The pink column represents a process of care with less than 25% compliance with the ABCDEF Bundle that was used in a 2008 mobility study.
The yellow column reflects the protocols and care provided in the TEAM Study, which were less than 50% in compliance with the ABCDEF Bundle.
And the green column represents the process of care that the patient in this case study actually received in an Awake and Walking ICU, which was 100% compliant with the ABCDEF Bundle.


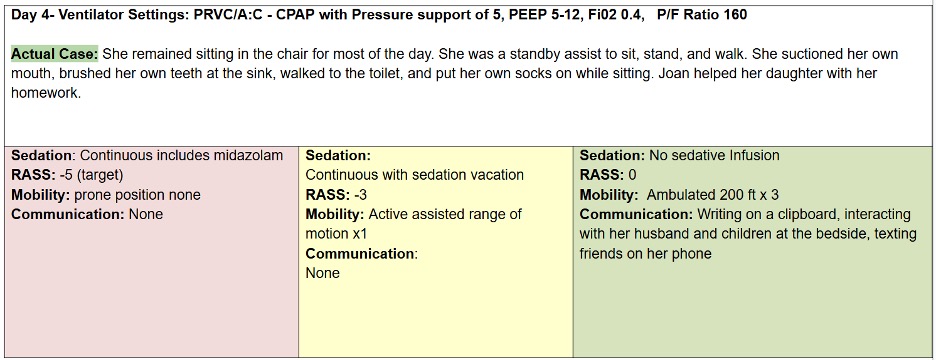
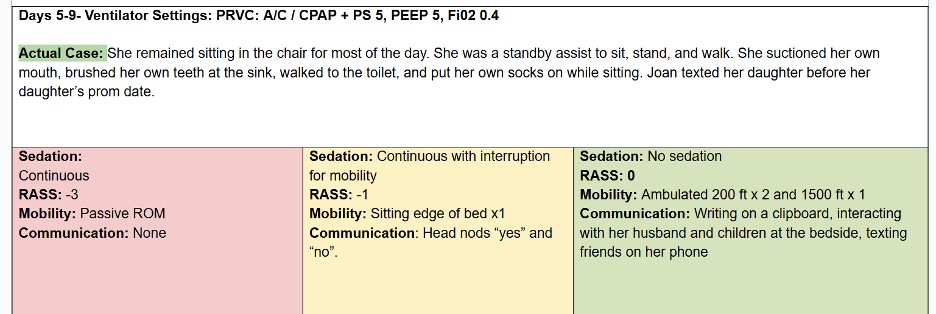
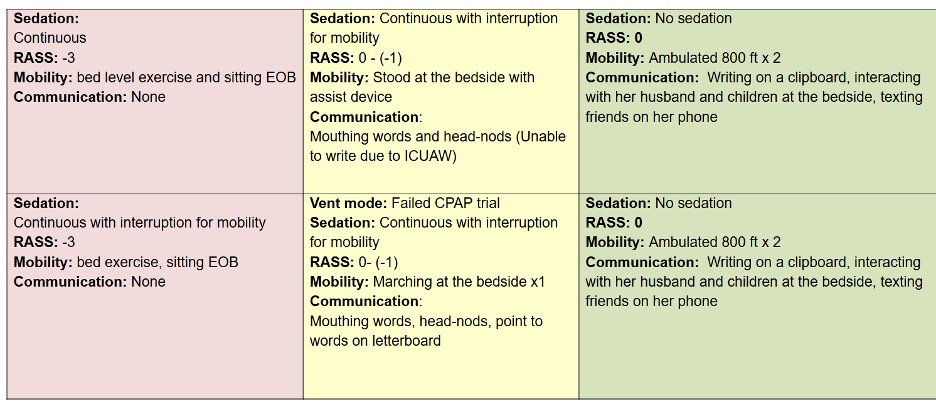
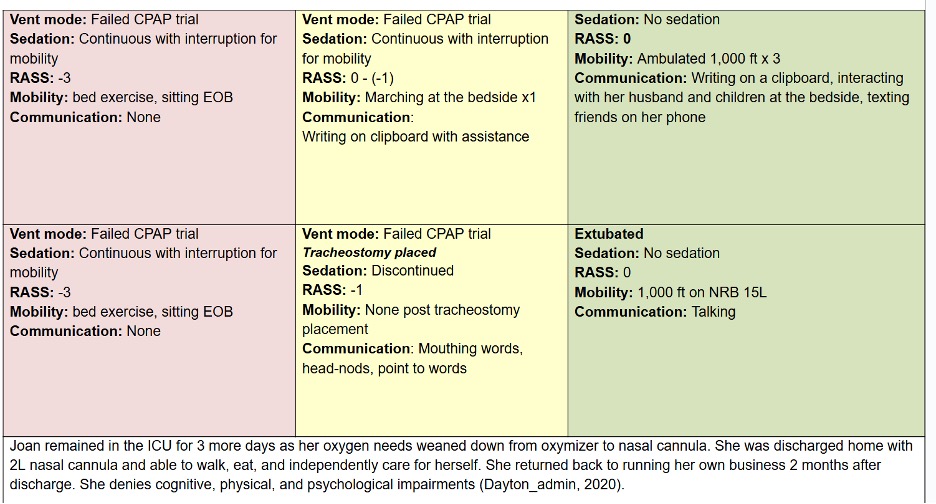
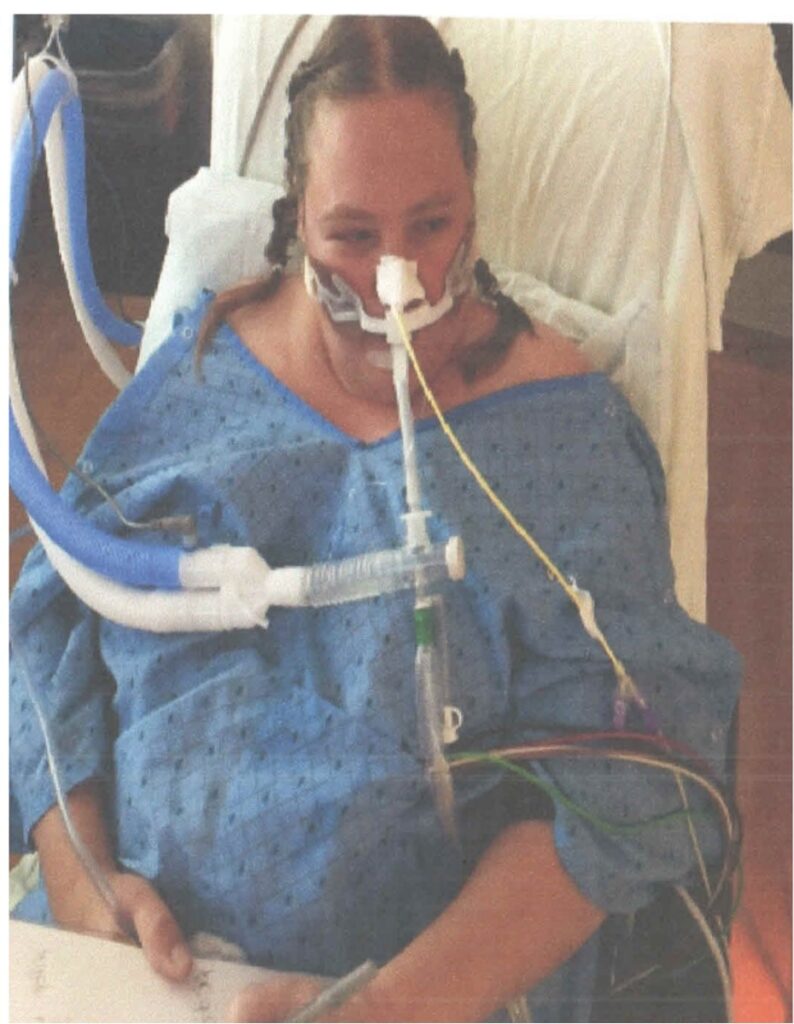
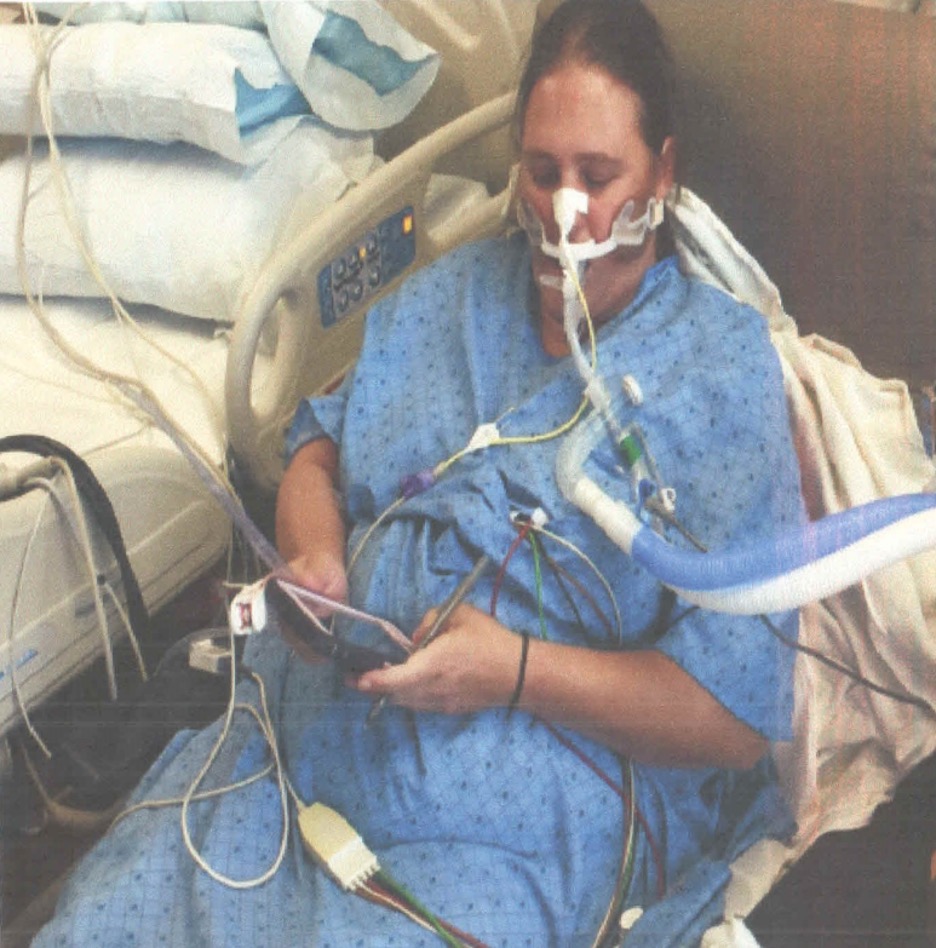
Joan was a 38-year-old woman with a history of tobacco use. She was admitted to a community hospital for pneumonia and intubated on day three, before being sent to an Awake and Walking ICU.
This survivor tells her story in Episode 17 of Walking Home From The ICU.
And if you’d like to learn more about what happened to Joan in the ICU, you can read my article: Joan’s Story: Yet Another Example of the Benefits of the ABCDEF Bundle.
Final Words
Despite its flaws, the TEAM Study is an important piece of research, as it helps us to identify and navigate barriers that are preventing us from mastering the ABCDEF Bundle and providing a protective and rehabilitative approach in the ICU.
But even the largest studies in the most credible of journals deserve a review beyond the abstract.
So, as we come to truly understand the objective and power of the ABCDEF Bundle, we should make a point of exploring research with discernment and striving to learn even more than what an abstract may tell us.
Want to learn how to implement and advocate for an Awake and Walking approach in your ICU? Contact me for more information or join our monthly meetings for ICU Revolutionists to gain support from fellow clinicians from around the world.